PRIASE 2021 guidelines for reporting animal studies in Endodontology. E xperimental animal studies should be evaluated as part of hazard characterization to ensure that adequate research has been carried out. Animal study experimental design.
Animal Study Experimental Design, Animal studies also test how safe and effective new treatments are before they are tested in peopleNCI Dictionary of Cancer Terms In Vitro Research In the laboratory outside the body. Initial Steps Literature Search A thorough search of the scientific literature must be per-formed to determine what is known about the focus of the study. The design choice of species vehicle route and timing of exposure conduct.
 Severity Classification From norecopa.no
Severity Classification From norecopa.no
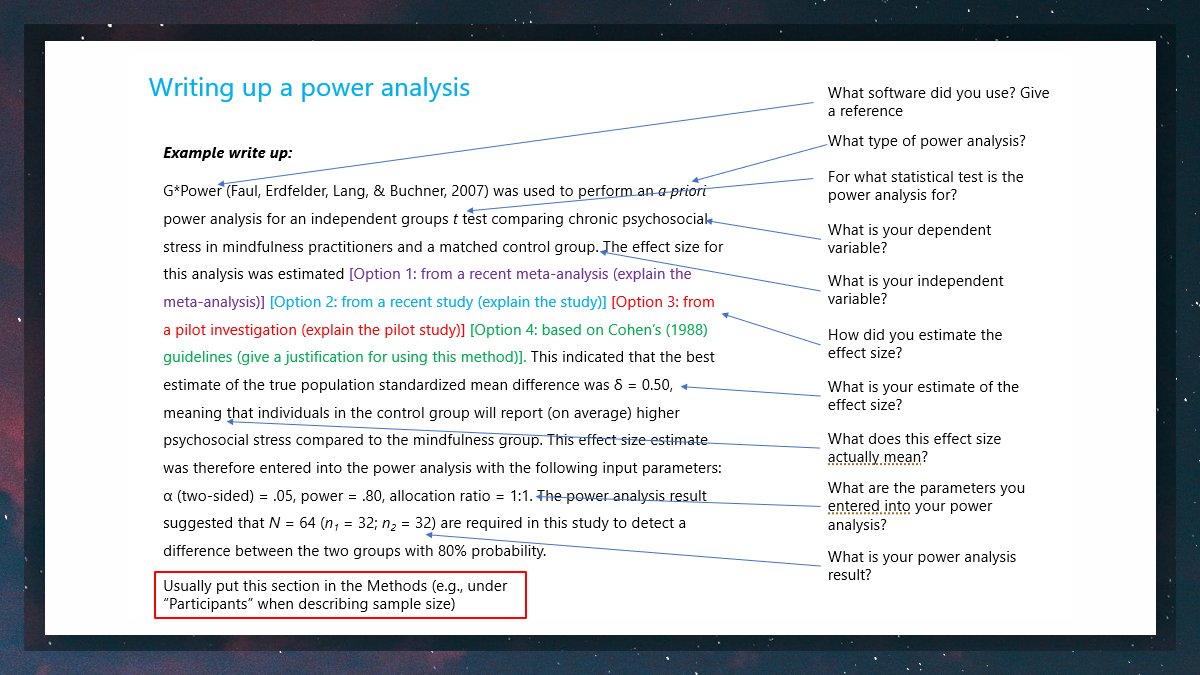
If done describe who was blinded and when. To date there are few studies examining the reporting of recommended experimental design feature to increase scientific rigor and reduce bias in animal experimental critical care research. Involved in the design of animal experiments and some practical information that should also be considered during this process. Details include animal characteristics eg species breed gender weight number of treatments number of experimental and sampling units arrangement of.
A consensus-based development 4 Consideration of Sex Differences in Design and Reporting of Experimental Arterial Pathology Studies-Statement From ATVB Council.
If done describe who was blinded and when. Randomly assigning animals to experimental groups is necessary to prevent bias that can arise when experimenters or pet owners are permitted to choose the treatment for each animal. Fundamental steps in experimental design for animal studies. In our study we evaluated the methodological quality of animal research in critical care journals in 2005 and 2015 and found a significant increase in the reporting of power analyses randomization and sample. Any steps taken to minimise the effects of subjective bias when allocating animals to treatment eg. Experimental design and reporting.
Read another article:

Fundamental steps in experimental design for animal studies. Fundamental steps in experimental design for animal studies. The number of experimental and control groups. Randomisation procedure and when assessing results eg. Research Animal Training An Interactive Resource Hub.
 Source: elsevier.com
Source: elsevier.com
Authors must provide details regarding the experimental design in a manuscript such that reviewers and readers have sufficient information about how the study was conducted and can evaluate the quality of the experimental design. Guidelines for experimental design and statistical analyses in animal studies submitted for publication in the Asian-Australasian Journal of Animal Sciences S. Animal studies continue to have a vital role in science development. Succinctly outline the formal scientific plan and direction for experimentation sequential description of procedures what will be done to the animals from obtain the animal to the end of study. Planning And Designing Research Animal Facilities 1st Edition.
 Source: abc10.com
Source: abc10.com
Animal studies also test how safe and effective new treatments are before they are tested in peopleNCI Dictionary of Cancer Terms In Vitro Research In the laboratory outside the body. For each experiment give brief details of the study design including. Any steps taken to minimise the effects of subjective bias when allocating animals to treatment eg. Experimental Design Analysis. Why Do We Experiment On Rats And Mice For Human Research Abc10 Com.
 Source: norecopa.no
Source: norecopa.no
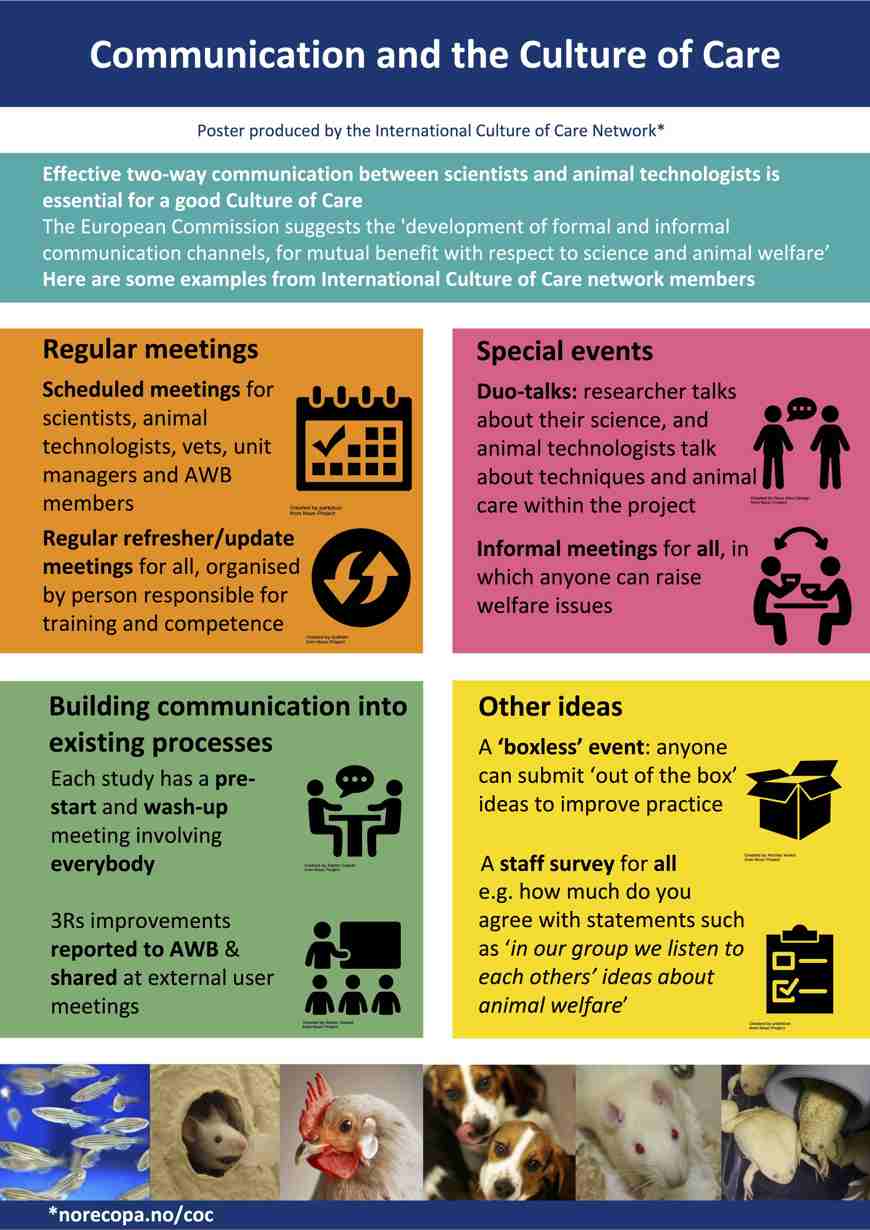
During the preparation of the study design investigators should hold his attention in planning important steps before starting the study. If a study is not designed to yield robust results and publications are not reported with enough. The opposite of in vivo in the body. Amid growing concerns about the poor design of animal experiments and its implications for the translatability of experimental results Michael F. Culture Of Care.
 Source: nature.com
Source: nature.com
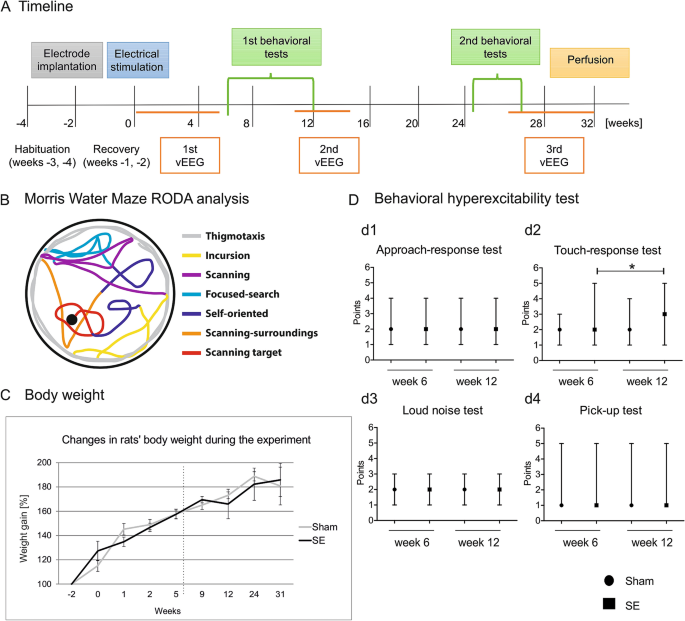
Authors must provide details regarding the experimental design in a manuscript such that reviewers and readers have sufficient information about how the study was conducted and can evaluate the quality of the experimental design. Any scientific technique or method should only be used when it is appropriate to what the researcher hopes to discover. Experimental Design Analysis. Succinctly outline the formal scientific plan and direction for experimentation sequential description of procedures what will be done to the animals from obtain the animal to the end of study. Behavioral Characteristics As Potential Biomarkers Of The Development And Phenotype Of Epilepsy In A Rat Model Of Temporal Lobe Epilepsy Scientific Reports.
 Source: uk.sagepub.com
Source: uk.sagepub.com
Any scientific technique or method should only be used when it is appropriate to what the researcher hopes to discover. These include the number of animals to be used pilot studies randomization blinding control groups type of variables collected and the statistical methods 149. Any steps taken to minimise the effects of subjective bias when allocating animals to treatment eg. During the preparation of the study design investigators should hold his attention in planning important steps before starting the study. The Design Of Animal Experiments Sage Publications Ltd.
 Source: nap.edu
Source: nap.edu
Authors must provide details regarding the experimental design in a manuscript such that reviewers and readers have sufficient information about how the study was conducted and can evaluate the quality of the experimental design. Randomly assigning animals to experimental groups is necessary to prevent bias that can arise when experimenters or pet owners are permitted to choose the treatment for each animal. Animal Research A laboratory experiment using animals to study the development and progression of diseases. E xperimental animal studies should be evaluated as part of hazard characterization to ensure that adequate research has been carried out. Workshop In Brief Reproducibility Issues In Research With Animals And Animal Models The National Academies Press.

Animal Research A laboratory experiment using animals to study the development and progression of diseases. Experimental Design Analysis. Details include animal characteristics eg species breed gender weight number of treatments number of experimental and sampling units arrangement of. Authors must provide details regarding the experimental design in a manuscript such that reviewers and readers have sufficient information about how the study was conducted and can evaluate the quality of the experimental design. Animal Models And Experimental Medicine.
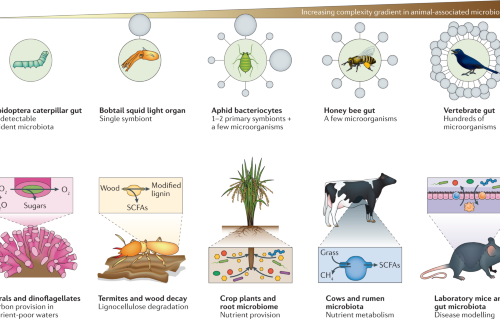 Source: nature.com
Source: nature.com
These include the number of animals to be used pilot studies randomization blinding control groups type of variables collected and the statistical methods 149. For each experiment give brief details of the study design including. Randomisation procedure and when assessing results eg. PRIASE 2021 guidelines for reporting animal studies in Endodontology. Study Designs.
 Source: animalresearch.info
Source: animalresearch.info
Initial Steps Literature Search A thorough search of the scientific literature must be per-formed to determine what is known about the focus of the study. During the preparation of the study design investigators should hold his attention in planning important steps before starting the study. Authors must provide details regarding the experimental design in a manuscript such that reviewers and readers have sufficient information about how the study was conducted and can evaluate the quality of the experimental design. These include the number of animals to be used pilot studies randomization blinding control groups type of variables collected and the statistical methods 149. Read The Four Main Reasons Why Animals Are Used In Medical Research.
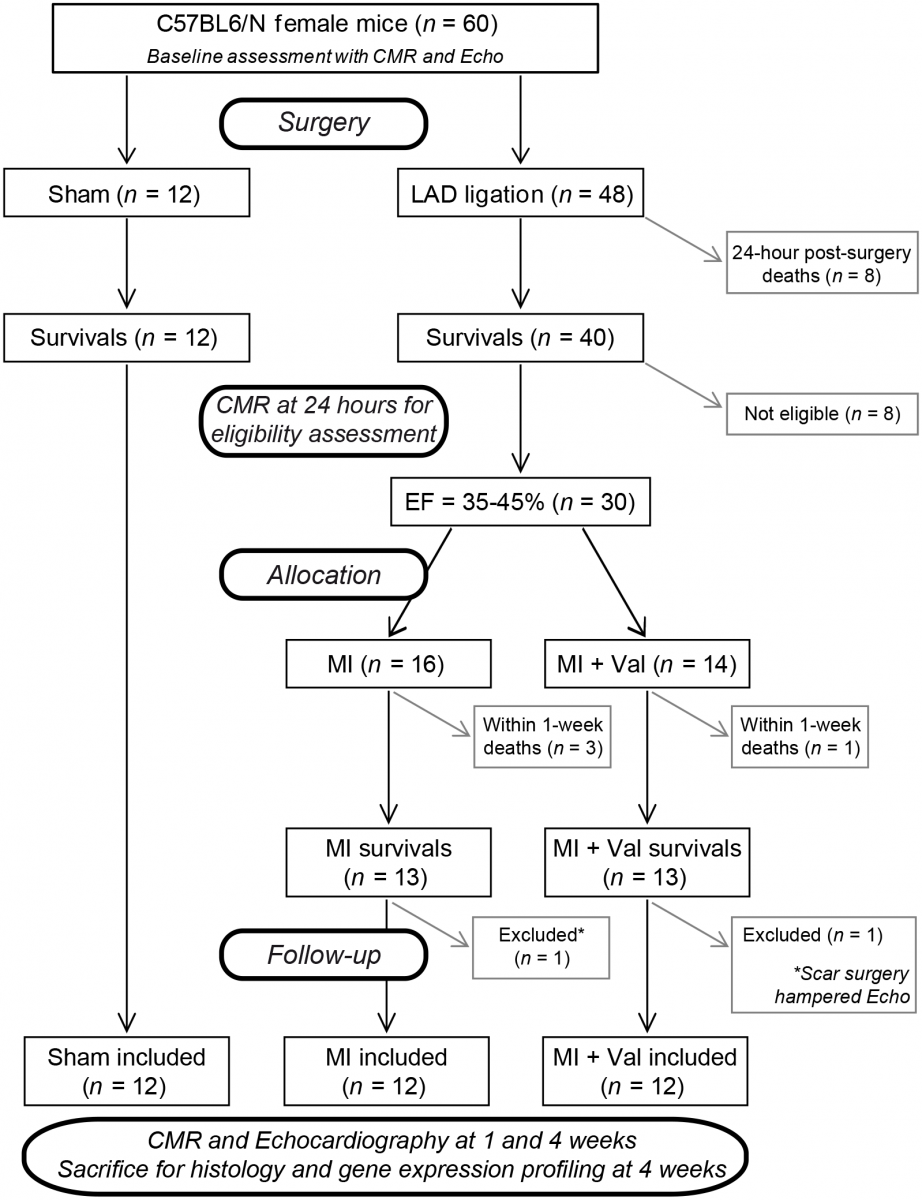 Source: arriveguidelines.org
Source: arriveguidelines.org
Experimental Design Analysis. Experimental designMaterials and Methods What will happen to the animals-Provide a completedescription of what will be done to the animals. Authors must provide details regarding the experimental design in a manuscript such that reviewers and readers have sufficient information about how the study was conducted and can evaluate the quality of the experimental design. Randomly assigning animals to experimental groups is necessary to prevent bias that can arise when experimenters or pet owners are permitted to choose the treatment for each animal. Inclusion And Exclusion Criteria Arrive Guidelines.
 Source: norecopa.no
Source: norecopa.no
Research on living animals is carried out when it will reveal information which cannot be obtained in other ways. Details include animal characteristics eg species breed gender weight number of treatments number of experimental and sampling units arrangement of. Authors must provide details regarding the experimental design in a manuscript such that reviewers and readers have sufficient information about how the study was conducted and can evaluate the quality of the experimental design. Experimental Animal and In Vitro Study Designs. Experimental Design And Reporting.

Fundamental steps in experimental design for animal studies. The aim of this review is to provide to new investigators an overview of the important steps involved in experimental designs and also to suggest some practical information that is commonly associated with this process. Seo Seoyoung Jeon. During the preparation of the study design investigators should hold his attention in planning important steps before starting the study. Research Animal Training An Interactive Resource Hub.

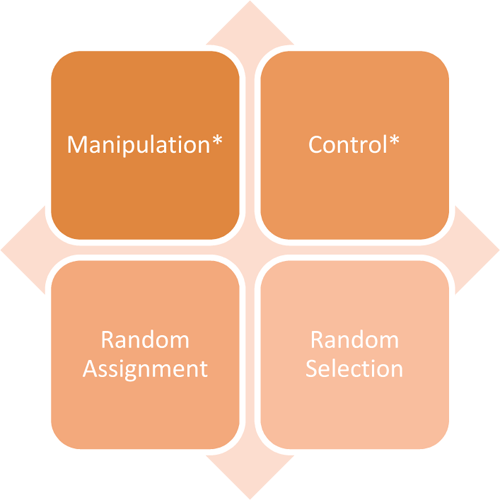
Details include animal characteristics eg species breed gender weight number of treatments number of experimental and sampling units arrangement of. The Design of Animal Experiments is intended for all research scientists who use laboratory animals with the aim of helping them to design their own experiments more effectively andor to improve their ability to communicate with professional statisticians when necessary. Experimental Design Analysis. Randomly assigning animals to experimental groups is necessary to prevent bias that can arise when experimenters or pet owners are permitted to choose the treatment for each animal. Study Design Arrive Guidelines.
 Source: ori.hhs.gov
Source: ori.hhs.gov
Research using animals in only legal in the UK and EU if there is no alternative method. Authors must provide details regarding the experimental design in a manuscript such that reviewers and readers have sufficient information about how the study was conducted and can evaluate the quality of the experimental design. Randomly assigning animals to experimental groups is necessary to prevent bias that can arise when experimenters or pet owners are permitted to choose the treatment for each animal. The number of experimental and control groups. Module 2 Research Design Section 2 Ori The Office Of Research Integrity.







